CPAP Lawsuits
In June 2021, the Food and Drug Administration (FDA) announced the recall of specific models of Philips Respironics (Philips) ventilators, bilevel positive airway pressure (Bilevel PAP, BiPAP, or BPAP) machines, and continuous positive airway pressure (CPAP) machines. The FDA issued the recall because of safety concerns and has labeled the recall as Class I, which is the most serious.
“The polyester-based polyurethane (PE-PUR) sound abatement foam, which is used to reduce sound and vibration in these affected devices, may break down and potentially enter the device’s air pathway. If this occurs, black debris from the foam or certain chemicals released into the device’s air pathway may be inhaled or swallowed by the person using the device,” the FDA said.
Since the FDA’s initial recall, information has linked the recalled devices with certain types of cancer and lung diseases. CPAP lawsuits are in their initial stages. CPAP lawyers are urging those who have used the devices for at least a year and have been diagnosed with these diseases to come forward. Once individuals come forward, scientific studies will begin to determine whether the diseases can be causally linked to the use of the devices.
Our Top Verdicts & Settlements
$12M
SETTLEMENT
Defective Product
$65M
SETTLEMENT
Defective Product
$13M
SETTLEMENT
Defective Product
What are CPAP and BPAP machines?
CPAP and BPAP machines help people breathe and are often used for patients who suffer from sleep apnea. BPAP machines pump air under pressure into the lungs. CPAP machines continually provide a stream of air through a mask, keeping the airways open. Continuous ventilators deliver a predetermined percentage of oxygen to help patients breathe.
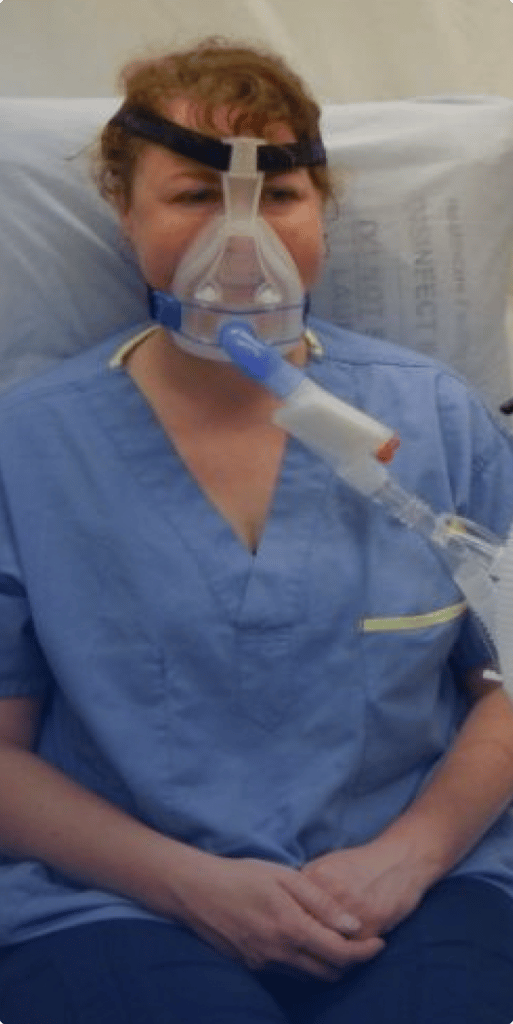
Which CPAP and BPAP machines were recalled?
The FDA recalled several CPAP and BPAP machines and told users of these machines to discuss alternative treatments with their physicians. The FDA lists the following recalled devices:
- E30 (Emergency Use
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C-Series ASV
- C-Series S/T and AVAPS
- OmniLab Advanced+
- SystemOne (Q-Series)
- DreamStation
- DreamStation Go
- Dorma 400
- Dorma 500
- REMstar SE Auto
- Trilogy 100
- Trilogy 200
- Garbin Plus, Aeris, LifeVent
- A-Series BiPAP Hybrid A30 (not marketed in the US)
- A-Series BiPAP V30 Auto
- A-Series BiPAP A40
- A-Series BiPAP A30
What are the potential adverse health effects of the devices?
The FDA requires manufacturers, such as Philips, to submit medical device reports (MDRs) when information suggests the devices may cause harm. Philipps submitted 30 MDRs between 2011 and April 2021, eight of which were from cases in the United States. None of the MDRs showed injury or death, the FDA said.
However, between April 2021 and April 2022, Philips, health care professionals, and consumers sent the FDA 21,000 MDRS, including 124 that reported deaths. The reports included instances of cancer, pneumonia, asthma, other respiratory problems, dizziness, infection, headache, chest pain, cough, dyspnea (difficulty breathing), and nodules. The FDA also lists the potential risks as headaches, inflammatory responses, hypersensitivity reactions, and toxic effects on the kidneys or liver.
The National Law Review notes that different types of cancer have varying latency periods and that research is continuing into causal links between the devices and certain cancers. “Right now, we believe the science is telling us that you will likely have to show at least one year of latency (a year passed between the time you first began using the recalled device and the time you developed an injury). After that, most forms of cancer will be reviewed by experts to determine a causal link,” the National Law Review says.
What should you do if you've used a recalled device?

You should first contact your health care provider first to see what alternative treatments are available, the FDA says. With the help of your provider, you also need to determine whether you’ve suffered injuries or illness because of using the machine. Many diseases may not necessarily present themselves immediately.

If you have been diagnosed with CPAP cancer or a lung injury and used one of the recalled devices for at least a year, you should contact a CPAP lawyer. Depending on the nature of the illness, you may be eligible to receive compensation to cover medical expenses, lost wages, lost earning capacity, and pain and suffering.
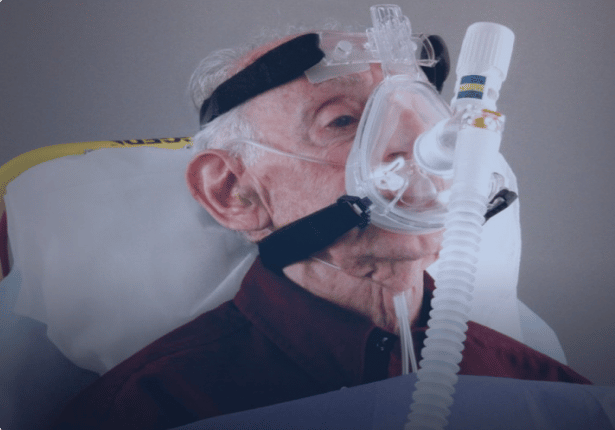
Our Sonstein law team is experienced in handling litigation similar to the CPAP lawsuit. We also understand the devastation of discovering you have gotten an illness due to using a device that was supposed to help you. Contact Sonstein Law today if you or a loved one has been diagnosed with a lung injury or cancer after using one of these devices.

Our Sonstein law team is experienced in handling litigation similar to the CPAP lawsuit. We also understand the devastation of discovering you have gotten an illness due to using a device that was supposed to help you. Contact Sonstein Law today if you or a loved one has been diagnosed with a lung injury or cancer after using one of these devices.

